--Powerful artificial intelligence combined with digital imaging designed to transform the screening process--
MARLBOROUGH, Mass.--(BUSINESS WIRE)--
Hologic, Inc. (Nasdaq: HOLX) announced today that its new Genius™ Digital Diagnostics System is now commercially available in Europe. The Genius Digital Diagnostics System is the next generation of cervical cancer screening that combines deep learning-based artificial intelligence (AI) with advanced volumetric imaging technology to help identify pre-cancerous lesions and cervical cancer cells in women. It was developed to provide actionable insights, and improve workflow and lab efficiency, all with one goal in mind – to eradicate cervical cancer.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20211104005111/en/
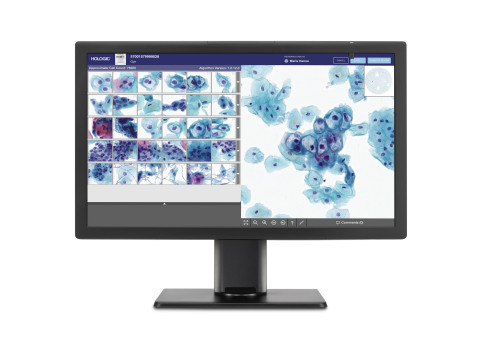
Genius™ Digital Diagnostics System for Cervical Cancer Screening
With the Genius Digital Diagnostics System, cutting-edge image analysis thoroughly interrogates every cell on a ThinPrep® Pap test image to curate a single view of the most clinically relevant objects. The new algorithm – GeniusTM Cervical AI – narrows tens of thousands of cells down to an AI-generated gallery, which arms healthcare providers with the critical information they need to guide earlier detection and better treatment decisions for the patients in their care.
“We strive to develop innovative solutions that meet our customers’ needs. We are delighted in the overwhelming positive feedback from the customers who have been the first in the world to use this technology,” said Andrew Pieprzyk, vice president, Strategic Development Diagnostics, International, Hologic. “The technology enhances the capabilities of individual laboratories and also has the potential to radically transform how cervical cancer screening is carried out, by enabling laboratories within the same network to collaborate across the world to manage the case load.”
Hologic has been working with leading laboratories in Europe to give them an early opportunity to evaluate the new system including ZotzKlimas Diagnostics Laboratories in Germany, where Founder, Dietmar Klimas, M.D., has led the review.
“As one of the largest labs in Germany, our main objective is to deliver accurate results to doctors and ultimately patients,” said Dr Klimas. “We were one of the first in the world to work with the Genius Digital Diagnostics system. The trial proved to me that this system can help my team to accurately process samples faster and more easily. We are continuing to use it following the trial.”
The Genius Digital Diagnostics System enables seamless and dynamic collaboration across laboratories within a network, connecting pathologists with remote review capabilities so each patient can benefit from the collective knowledge of geographically dispersed experts. Digital case review promises to enhance the experience for lab partners by improving workflow and accelerating review time.
Hologic now offers the first CE-marked comprehensive cervical cancer screening portfolio from sample collection to digital diagnosis.1 The Genius Digital Diagnostics System consists of the GeniusTM Digital Imager for image acquisition, the GeniusTM Cervical AI algorithm for analyzing images, the GeniusTM Image Management Server (IMS) for storing images and the GeniusTM Review Station for case review. The complete system is scalable, designed to fit the present and future needs of laboratories.
About Hologic
Hologic, Inc. is an innovative medical technology company primarily focused on improving women’s health and well-being through early detection and treatment. For more information on Hologic, visit www.hologic.com.
Forward-Looking Statements
This press release may contain forward-looking information that involves risks and uncertainties, including statements about the use of Hologic’s Genius™ Digital Diagnostics system. There can be no assurance these products will achieve the benefits described herein or that such benefits will be replicated in any particular manner with respect to an individual patient. The actual effect of the use of the products can only be determined on a case-by-case basis depending on the particular circumstances and patient in question. In addition, there can be no assurance that these products will be commercially successful or achieve any expected level of sales. Hologic expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any such statements presented herein to reflect any change in expectations or any change in events, conditions or circumstances on which any such statements are based.
Hologic, Genius, ThinPrep, and The Science of Sure and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries.
SOURCE: Hologic, Inc.
_________________
1 Hologic, Inc. Data on File.

View source version on businesswire.com:
https://www.businesswire.com/news/home/20211104005111/en/
Investor Contacts
Michael Watts
Vice President, Investor Relations and Corporate Communications
+1 (858) 410-8588
michael.watts@hologic.com
Ryan M. Simon
Vice President, Investor Relations
(858) 410-8514
ryan.simon@hologic.com
Media Contact
Jane Mazur
Vice President, Divisional Communications
+1 (585) 355-5978
jane.mazur@hologic.com
Source: Hologic, Inc.